This article traces the history of the atom from ancient times to modern atomic models, including the Greek concept of the monad. It highlights the different stages of development in atomic models, culminating in the current atomic geometry model.

Overview
The idea of the atom has evolved over time, with contributions from ancient civilizations and scientists. The concept originated in ancient India and Greece and progressed through philosophical and scientific discoveries. Key milestones include the identification of electron shells, the realization of electrons as both particles and waves, and the development of the current model of the atom. By studying the geometry and patterns of atoms, we can unlock the secrets of natural processes and live in harmony with our environment.
First notions of the atom
The concept of the atom has evolved over centuries, with ancient civilizations and notable scientists contributing to our understanding of its components and spatial configuration. In this blog post, we will delve into the fascinating history of the atom, exploring its origins and the numerous scientific breakthroughs that have shaped our knowledge of this fundamental unit of matter.
The journey begins in ancient India, where philosopher Archarya Kanada, in the 6th century BC, speculated that matter consisted of minute particles called the Iota. Kanada’s profound observation laid the foundation for the concept of the atom. Fast-forward to Ancient Greece, where philosophers Leucippus of Miletus and Democritus of Abdera coined the term “Atomos,” from which we derive the modern word “atom.” Their ideas expanded on the notion put forth by the Indian philosophers.
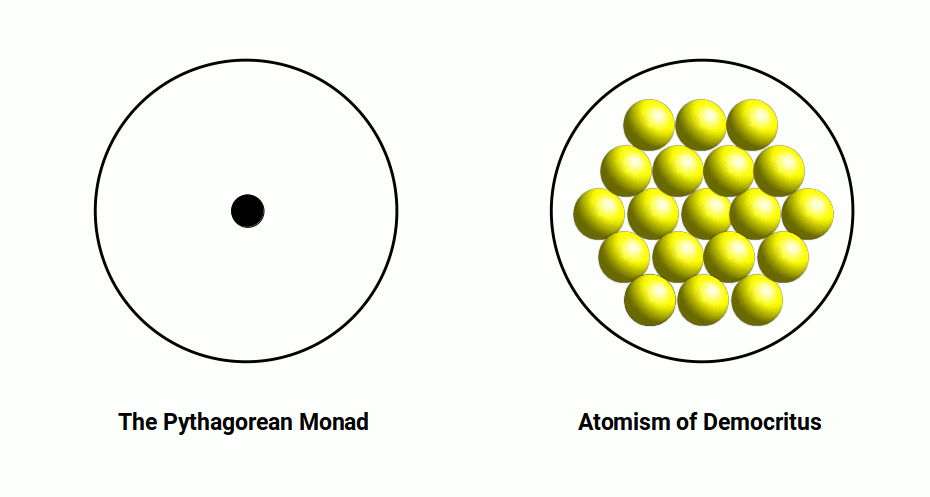
The exploration of the atom took a metaphysical turn within Sacred Geometry, with the introduction of the concept of the Monad, akin to the God Particle. However, it was the discovery of the electromagnetic field that truly revolutionized our understanding. The realization of this new force, capable of producing light and moving machines, prompted extensive study throughout the 19th century.
First models of the atom
Chemist John Dalton, followed by J.J. Thomson, made significant contributions to the development of models representing the atom. Dalton’s Solid Sphere model provided an initial understanding of the atom’s structure, which was further expanded upon by Thomson’s Plum Pudding model. This model depicted the atom as a spherical mass of matter with embedded electrons, resembling our current understanding of the Big Bang Theory.
Ernest Rutherford’s famous gold foil experiment in 1911 shattered this contemporary perception. By observing alpha particles being reflected, Rutherford proposed the existence of the atomic nucleus, introducing the concepts of protons and electrons. This discovery led to the acceptance that electrons orbit a central nucleus, creating electron shells that are quantized into energy levels.
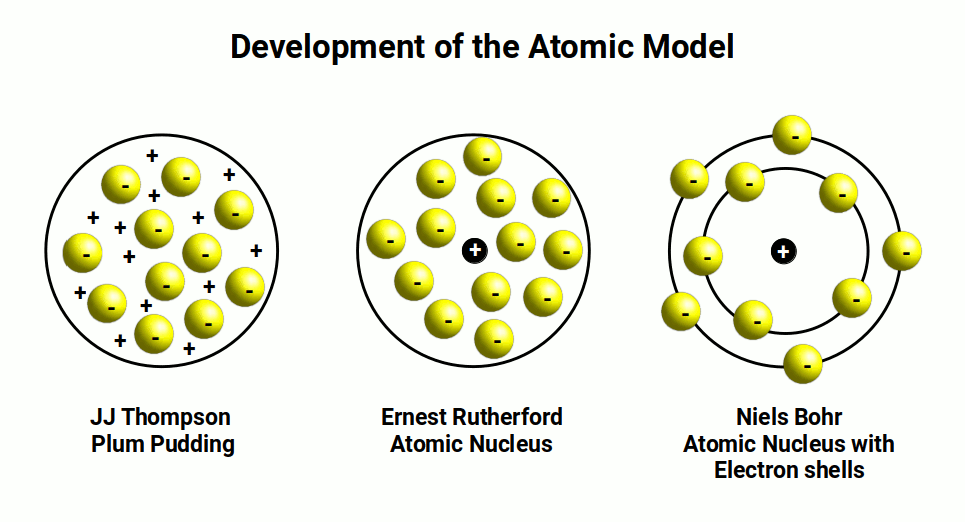
The mechanism by which electrons transition between shells, releasing quantized energy, perplexed scientists for some time. Albert Einstein’s establishment of the photon as a quantized wave packet of energy, later verified by Max Planck, provided crucial insights into this phenomenon. In 1913, Niels Bohr developed the Planetary model, which likened the atom’s structure to our solar system. This model depicted protons at the center, surrounded by electron orbits, and remains the prevailing model used in chemistry to this day.
First notions of the atom
Whilst the Bohr model had revealed the structural nature of the electron cloud, one major question remained unanswered: why didn’t electrons lose energy and collapse into the nucleus? The concept of the electron as a wave function emerged, which Louis de Broglie’s Pilot Wave model sought to explain. These developments challenged the prevailing belief that electrons were purely particles, revealing their wave-like properties, similar to light.

An experimental example of the Piolet wave model of the electron, guided by a ‘Matter Wave’.
Heisenberg’s Uncertainty Principle, rooted in matrix theories, expanded upon these findings by proposing a probability function for electrons. This interpretation, known as the Copenhagen interpretation, posits that an electron exists in all its possible states simultaneously until measured.
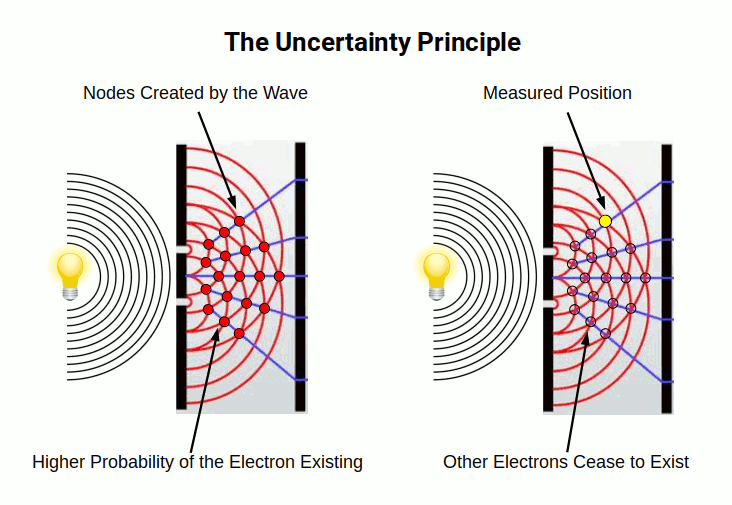
Wave equations
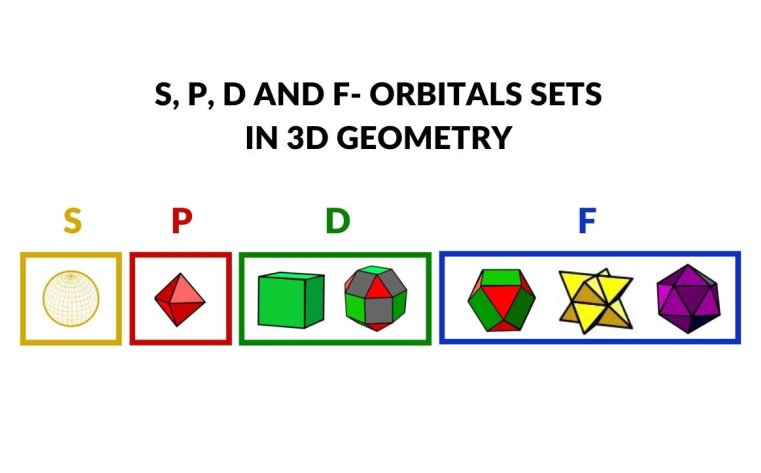
Erwin Schrödinger’s equations in the 1920s collapsed the electron cloud into specific orbitals known as S, P, D, and F, giving further insights into the atom’s organization. This interpretation, based on probabilities and quantized orbits, has endured and remains the foundation of our understanding today.

Wave predictions generated by the Schrödinger Equations
Throughout these discoveries, the atom’s geometric nature became evident. While most of an atom is composed of empty space, comprehending this space allows us to gain a deeper understanding of the universe.
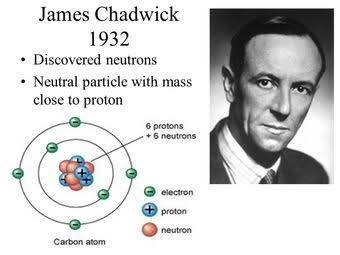
Discovery of the neutron
In the 1930s, the discovery of the neutron through precise measurements added another intriguing element to the atom’s composition. This neutron conundrum was solved by James Chadwick in 1932. This led to the development the 1st nuclear weapon in world was II along with subsequent developments during World War II.
Wave predictions generated by the Schrödinger Equations
Atomic Geometry
While the Copenhagen interpretation of the atom persists in mainstream academia, alternative perspectives that focus on geometric patterns emerge as well. These patterns, when observed through the lens of space, provide a clearer understanding of the periodic table’s cohesive geometry and its alignment with the natural forms produced by our planet.
Atomic Geometry is the worlds first model of the atom that can predict the radii of all stable elements.
By delving into the atomic patterns of nature, we can unlock the secrets necessary to harness nature’s abilities, such as splitting water, creating sustainable energy, and living harmoniously with our environment.
THE
Conclusion
In conclusion, the concept of the atom has transformed throughout history, thanks to the contributions of ancient philosophers and pioneering scientists. From the ancient Greeks to modern-day quantum mechanics, our understanding has evolved, unravelling the atom’s intricate structure and the role of probability within its realm. As we continue to explore the geometric patterns and energetic potential of atoms, we move closer to a more profound understanding of our universe and our place within it.
Carry On Learning
This article is part of our new theory, Atomic Geometry
browse more interesting post from the list below

D-Orbital Geometry – Part 2
The 2nd set of D-orbitals contain various anomalies that are explained by the Geometric model of the atom. Part 2 of 3.
The 4D Electron Cloud
The electron cloud is normally thought about in terms of the probability of the particle. However, our notion of a 4D Aether dispenses with this notion, returning a sense of normally to atomic physics.

P-orbital Geometry
P-orbitals form in sets of 6 producing an octahedral structure. By producing this form based on the average radii for each set, we can approximate the radius for almost all elements on the periodic table.


